News

Harvard Researchers Validate Metalens-Based QPI Endoscopy Using Phasics SID4-HR
Oct. 16, 2025
Why phase matters in endoscopy
In biomedical imaging, many optical techniques are capable of visualizing semi-transparent or weakly scattering tissues, but they often rely on exogenous contrast agents such as fluorescent dyes or labels. Quantitative Phase Imaging (QPI) offers a powerful alternative by providing intrinsic, label-free contrast that reflects variations in refractive index and thickness within the sample. This makes QPI particularly effective for thin and weakly absorbing specimens, where traditional amplitude-based microscopy fails to yield sufficient structural information.
Endoscopy, by contrast, is designed for in vivo imaging of thick, highly scattering tissues, where light propagation becomes dominated by multiple scattering and absorption. Under these conditions, recovering quantitative phase information is considerably more challenging. Nevertheless, recent advances have demonstrated that QPI principles can be integrated into endoscopic systems through innovative optical and computational approaches.
Although imaging thick and diffusive tissues remains a persistent challenge, not only for QPI but for biomedical imaging in general, combining phase-based contrast with endoscopy represents a promising direction toward quantitative, minimally invasive optical diagnostics.
A recent study from Harvard University and Massachusetts General Hospital, published in Light: Science & Applications, demonstrates a compact, interference-free route to quantitative phase imaging intrinsically compatible with endoscopy. The approach leverages a metalens whose focal length shifts with wavelength, under broadband illumination, a color camera’s RGB channels correspond to distinct defocus planes along the optical axis, an effect known as chromatic focal shift.
By integrating this multi-plane information within the Transport of Intensity Equation (TIE) framework, the researchers reconstructed quantitative phase maps directly from a single color image, without any interferometer or mechanical scanning.
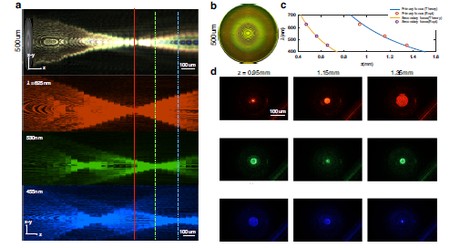
Figure 1 (a) Using red (625 nm), green (530 nm), and blue (455 nm) LEDs, the longitudinal scan shows distinct focal positions: red focuses near 0.95 mm, green at 1.15 mm, and blue at 1.35 mm, with a weak secondary focus for blue and green at 0.5 mm. (b) Microscope image of the metalens surface morphology. (c) Measured focal length versus wavelength, in good agreement with the theoretical λ/πr² = z relation. (d) Two-dimensional point-spread functions (PSFs) for RGB channels measured on a standard microscope. Image source: Light: Science & Applications, “Quantitative phase imaging endoscopy with a metalens.”
Unlike conventional multi-frame or interferometric QPI methods, this strategy retrieves the phase from a single color exposure. It removes the need for additional reference optics or mechanical movement, simplifying the optical design while preserving the quantitative nature of phase imaging.
Calibration and validation with PHASICS: from test targets to biological samples
Before applying the technique to living specimens, the researchers validated its performance using a Siemens star phase target. This allowed them to test the system under well-defined conditions: they computed the contrast-transfer function (CTF) to quantify spatial resolution, and compared the reconstructed phase with a reliable reference to assess accuracy.
For the reference, they used a Phasics SID4-HR quantitative phase imaging camera, chosen for its high sensitivity, stability, and well-established quantitative precision. The SID4-HR has been widely adopted by research groups worldwide as a ground-truth instrument for phase measurements, making it an ideal benchmark for this study.
In practice, the SID4-HR was attached to the port of a conventional transmission microscope to record the same Siemens target. The resulting reference phase map was then compared point by point with the reconstruction obtained using the metalens method.
The comparison confirmed that the new approach retrieves height variations with an accuracy of approximately 0.1 λ and achieves a lateral resolution close to 1 μm, sufficient to capture cell-level structures.
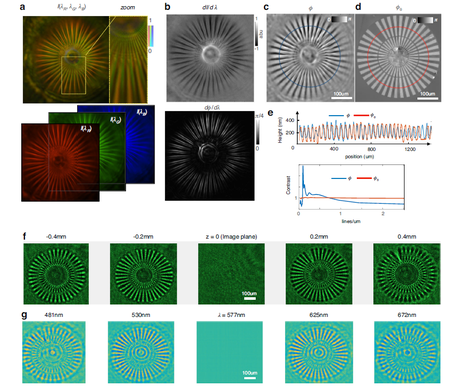
Figure 2 Quantitative phase imaging of a Siemens star target using the metalens (target thickness 250 nm, refractive index 1.52).
(a) Image acquired by a color camera, separated into RGB channels by filters.
(b) The intensity differences among the three channels (dI/dλ) correspond to effective intensity variation. After QPI reconstruction, the phase variation with wavelength (dφ/dλ) reveals the spatial phase gradient.
(c) The reconstructed QPI image provides accurate measurement of the target height.
(d) The benchmark measurement was obtained with a Nikon microscope and a commercial phase imaging camera (Phasics SID4-HR).
(e) Tangential comparison between the reconstruction (blue) and the benchmark (red) shows good agreement after conversion to equivalent height. The contrast transfer function indicates a lateral resolution better than 1 μm.
(f) Intensity images acquired experimentally at different defocus values.
(g) A spectral stack computed from a single color image matches the experimental defocus images, validating the accuracy of the phase reconstruction.
Image source: Light: Science & Applications, “Quantitative phase imaging endoscopy with a metalens”.
To further test real-world feasibility, the team mounted the metalens directly at the tip of a coherent fiber bundle (CFB). In typical conditions, fiber bundles disrupt spatial phase coherence, making QPI extremely difficult. In this configuration, however, the phase information was pre-encoded into intensity through the chromatic defocus among RGB channels. As a result, the phase could still be faithfully recovered after transmission through the fiber bundle.
Under these conditions, the team imaged Spirogyra samples and clearly visualized subcellular structures such as spiral chloroplast bands and the relative depth of overlapping filaments. The experiment demonstrated that the method remains effective in realistic endoscopic scenarios.
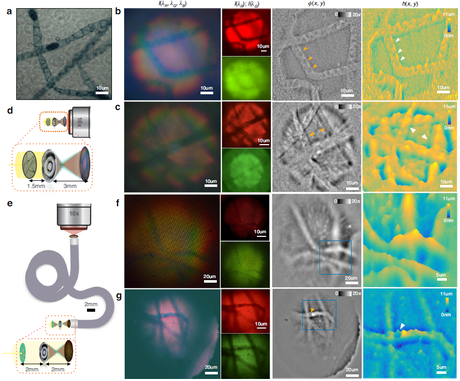
Figure 3 QPI of a Spirogyra sample.
(a) Reference image showing spiral chloroplasts and dark bead-like gametangia.
(b, c) From left to right: white-light color image, single-channel images, reconstructed phase map, and height map (metalens direct-coupled configuration). The single-shot QPI distinctly resolves chloroplast bands and opposite phase curvature across the focal plane.
(d) Metalens direct-coupled configuration at 2× magnification.
(e) Configuration combining the metalens with a coherent fiber bundle at 1×.
(f, g) QPI through the fiber endoscope distinguishes overlapping filaments and reveals individual gametangia even under low illumination.
Image source: Light: Science & Applications, “Quantitative phase imaging endoscopy with a metalens.”
Assessing the reliability of PHASICS quantitative measurements
Whenever a new imaging technique is introduced, a fundamental question arises: Can the quantitative results be trusted?
This is a question faced by nearly every development team:
- Is the reconstructed phase truly accurate?
- How can I prove that the results are reliable?
- Is there an established instrument that can serve as a validation reference?
In this work, the researchers’ decision to include a recognized quantitative reference provides that assurance. By comparing their new approach with an independently validated system, they avoided the pitfall of self-validation and reinforced the scientific credibility of their results.
Here, the Phasics SID4-HR served as the trusted benchmark. It was not meant to replace the new technique, but to anchor it to a known quantitative standard, enabling the findings to be confidently accepted by the broader research community.
We sincerely thank the Harvard and Massachusetts General Hospital team for their outstanding contribution to advancing quantitative phase imaging and for trusting Phasics technology as a reliable reference in their work.
At Phasics, we continue to support the research community with quantitative imaging tools that enable next-generation optical diagnostics.
📌 For more insights into cutting-edge optical research, follow our official website and explore the studies of featuring Quantitative Phase Imaging (QPI).
Reference
Shanker, A., Fröch, J.E., Mukherjee, S., Zhelyeznyakov, M., Brunton, S.L., Seibel, E.J. & Majumdar, A.
Quantitative phase imaging endoscopy with a metalens.
Light: Science & Applications 13, 305 (2024). https://doi.org/10.1038/s41377-024-01713-9
Search
Categories
订阅Phasics资讯获取最新信息
Join us on Wechat
