Phasics
- Wavefront, MTF and QPI measurement solutions
- Products
- Applications
- Markets
- Company
- Contact us

Aug. 22, 2025
In tumor immunology, accurately identifying T-cell-mediated cytotoxicity against tumor cells is essential for evaluating the efficacy of immunotherapy. Conventional approaches primarily rely on indirect indicators such as cytokine secretion, apoptosis markers, or endpoint metabolic analyses. While these methods provide insight into global immune activity, they fail to capture the dynamic behavior of individual immune cells, especially under complex co-culture conditions and lack real-time, label-free, and continuous monitoring capabilities.
To address this challenge, Teitell Lab at the University of California, Los Angeles (UCLA) developed a novel analytical platform combining Quantitative Phase Imaging (QPI) with machine learning. This platform enables the label-free, single-cell-resolved, real-time tracking and classification of T cell-induced cytotoxic events against tumor cells.
Imaging Core: Live Cell Interferometry Powered by Phasics QPI
At the heart of this methodology is Live Cell Interferometry (LCI), enabled by the Phasics SID4-Bio, a quantitative phase imaging camera based on Quadriwave Lateral Shearing Interferometry (QWLSI). The system captures real-time phase maps using a high-framerate, label-free interferometric imaging setup during co-culture experiments, allowing extraction of time-resolved features such as cell dry mass, morphology, and motility.
From Feature Extraction to Cytotoxic Event Recognition
The team applied this system to a co-culture model of M202 melanoma cells and F5 TCR-transduced T cells. Using an automated pipeline, individual cells were segmented and tracked across time, and over ten physical and biological features were extracted from each trajectory, including dry mass change , cell area, contour length, displacement, and phase contrast. These features were structured into time-series datasets with sliding window transformations, forming the input matrix for machine learning classifiers.
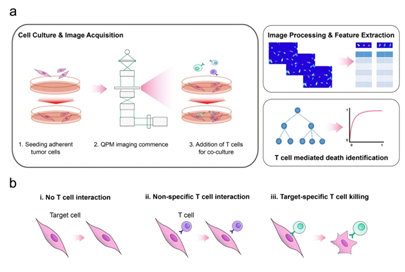
Figure 1. Experimental setup, data analysis pipeline, and T cell–target cell interaction modes. Image adapted from Kim D.N.H., Lim A.A., Teitell M.A., Sci Rep 11, 19448 (2021).
QPI images captured by the SID4-Bio under a 20×/0.4 NA objective at 10 frames per second covered 60 wells, with continuous recording lasting 8–12 hours per run. The results revealed distinctive temporal patterns in phase density and derived features between cytotoxic and non-cytotoxic events.
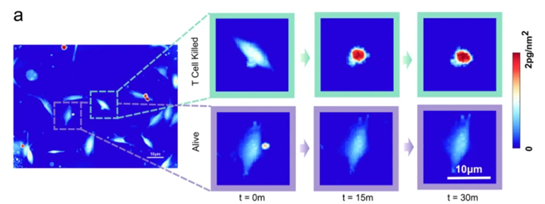
Figure 2. Phase density variation and temporal features of T-cell-mediated killing events, Image adapted Kim D.N.H., Lim A.A., Teitell M.A., Sci Rep 11, 19448 (2021).
Machine learning models, including Naive Bayes, Random Forest, and Support Vector Machines, were evaluated. The best performing classification accuracy is obtained with Random Forest model using all the features (cf Figure 3). By analyzing the variable of “area under the curve” (AUC) of the receiver-operator characteristics (ROC) curve as a reliable metric for classification accuracy, the best performing Random Forest model has a AUC of 0.9665.
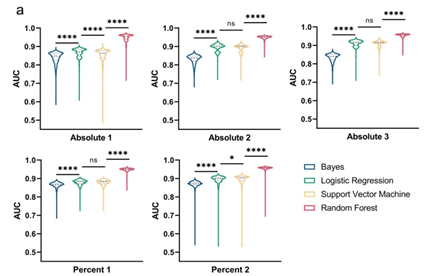
Figure 3: ROC curves in rare event detection across systems, Image adapted from Kim D.N.H., Lim A.A., Teitell M.A., Sci Rep 11, 19448 (2021).
The trained model was directly transferred to an entirely different setup: M257 melanoma cells co-cultured with NY-ESO-1-specific TCR T cells. Even under simulated rare event scenarios (positive event ratios ranging from 1:1 to 1:100,000), the model maintained an AUC > 0.95, without retraining, demonstrating excellent generalization across systems.
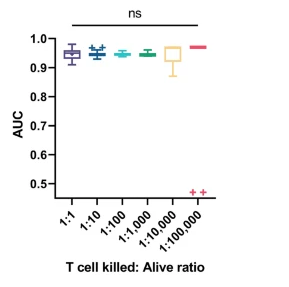
Figure 4: Classification performance (AUC) across 30 random datasets at different T cell kill-to-alive ratios. Outliers in 1:100,000 arise from a single killing event misclassified as alive. Image adapted from Kim D.N.H., Lim A.A., Teitell M.A., Sci Rep 11, 19448 (2021).
Why Phasics QPI Was Crucial
The Phasics SID4-Bio was a key component for image and data acquisition in this work . Integrated with a Zeiss Axio Observer A1 inverted microscope and CO₂-compatible multiwell stage-top incubator, it enabled 8–12 hours of stable, non-invasive imaging using white light, no fluorescence, no laser, and no phototoxicity.
Its wide field-of-view and vibration resistance allowed high-throughput acquisition across multiple positions. The rich physical data acquired particularly dry mass and morphological dynamics, formed the critical basis for machine learning classification of immune responses.
While the full imaging and analysis pipeline was custom-built by the researchers, the core quantitative data enabling event discrimination originated from the Phasics QWLSI technology-based SID4-Bio camera.
Thanks to the UCLA research team for their valuable contributions and technical support throughout the study. This work demonstrates how label-free, continuous QPI data can serve as robust inputs for machine learning, enabling generalizable classification of functional immune responses at single-cell resolution. It highlights the growing role of QPI, especially Phasics’ technology, in advancing immuno-oncology, cell-based drug screening, and mechanistic immunology.
If you're interested in exploring how QPI can support your research, feel free to contact the Phasics team.
👉 [Click here] to discover how our QPI solutions can enhance your high-content imaging workflows.

Phasics SID4-sC8 QPI Camera

Phasics SID4-Bio QPI Camera
Reference
