Phasics
- Wavefront, MTF and QPI measurement solutions
- Products
- Applications
- Markets
- Company
- Contact us

April 25, 2025
In a recent study published in the American Journal of Physiology - Cell Physiology, researchers from the PRETI Institute (Physiopathology and Regulation of Ionic Transport) at the University of Poitiers developed primary human cell models of meibomian gland epithelial cells (hMGECs) and conjunctival epithelial cells (hConECs).
Their work provides new insights into how key ion channels—including ENaC, CFTR, and TMEM16a—and aquaporins (AQP3, AQP5) coordinate to regulate tear film homeostasis.
By integrating transmembrane current recordings, calcium imaging, and label-free Quantitative Phase Imaging (QPI), the team demonstrated the pivotal roles of cAMP and purinergic signaling in controlling chloride secretion and water transport.
These findings establish a valuable in vitro platform for advancing research into ocular surface diseases such as dry eye syndrome.
SID4 Bio: A Core Enabler of the Study
Phasics' SID4 Bio QPI camera was instrumental to the study’s success.
Leveraging its patented Quadriwave Lateral Shearing Interferometry (QWLSI) technology, SID4 Bio enabled real-time, label-free, and non-invasive measurement of water flux across epithelial membranes—capturing dynamic processes that are otherwise extremely difficult to monitor.
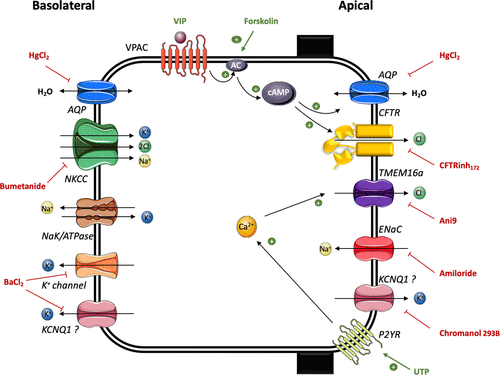
Figure 1. Schematic of ion and water transport pathways in hConEC and hMGEC models.
Upon stimulation with the cAMP activator forskolin (10 μM), SID4 Bio detected significant decreases in optical path difference (OPD), indicating water influx:
These results confirmed a cAMP-dependent increase in water permeability in both cell types.
Further experiments using mercury chloride (HgCl₂, 5 μM) verified that this effect was primarily mediated by mercury-sensitive aquaporins.
Western blot analysis also confirmed the expression of AQP3 and AQP5, providing strong functional and molecular evidence for aquaporin-driven water transport.
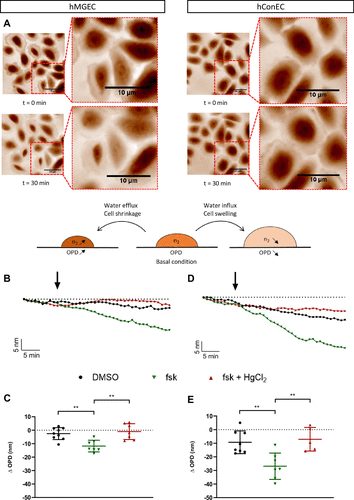
Figure 2. Representative phase images of hConEC cells before (t = 0 min) and after (t = 30 min) forskolin stimulation. OPD traces and bar graphs illustrate the effects of forskolin and HgCl₂ treatments on hMGEC and hConEC cells. (Statistical analysis: ANOVA and Tukey post hoc test, P < 0.01).
Why Scientists Trust SID4 Bio for Life Sciences Research
The SID4 Bio system empowers researchers to capture fine biological dynamics with unmatched precision and clarity:
🔬 Label-free and non-invasive: Observe real-time cellular responses without the need for dyes or labels, preserving native cell function.
📏 Nanometric sensitivity: Detect minute changes in cell dry mass, volume, and membrane dynamics with sub-nanometer axial resolution.
⚙️ Seamless integration: Quickly plug into standard inverted or upright microscopes, enabling fast setup and flexible experimental workflows.
📊 Reliable, quantified results: Built-in analysis modules ensure reproducible measurements of cell mass, volume, and mechanical properties.
🌈 Integrated phase-fluorescence imaging: Overlay phase and fluorescence data in one click to enrich phenotypic characterization.
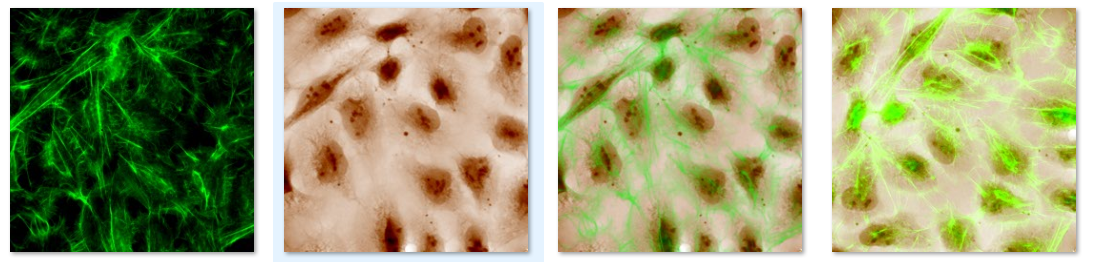
Figure 3. SID4 Bio seamlessly combines phase and fluorescence imaging for enhanced analysis.
📖 Read the full article here, click here to see more microscopy applications.
📩 Request a live demonstration to see how SID4 Bio captures life at the nanometric scale with precision and ease.
Reference:
Radji C, Barrault C, Flausse R, Leveziel N, Cantereau A, Bur C, Terrasse G, Becq F.
Modeling ocular surface ion and water transport by generation of lipid.
Am J Physiol Cell Physiol. 2024;328(3). DOI: 10.1152/ajpcell.00560.2024
